Abstract
Autologous hematopoietic stem cell transplantation (ASCT) is recommended for transplant-eligible pts (pts) with newly diagnosed multiple myeloma (MM) as well as AL Amyloidosis (AA). However, melphalan-associated gastrointestinal (GI) and cardiac toxicities may limit its applicability. GI toxicity is a main limitation to ASCT in the elderly population which constitute a significant proportion of MM pts, where the median age at diagnosis is 68 years. There is an unmet need for measures to minimize non-hematological toxicities without compromising the melphalan (MEL) anti-tumor efficacy; this could lead to expansion of transplant eligibility to older pts as wells pts with high-risk cardiac AA. Also, minimizing treatment-related morbidity may allow dose-escalation or the addition of novel anti-myeloma agents to MEL conditioning; such may improve the depth of response and remission duration after ASCT. Here, we report outcomes of an integrated ASCT program for high-risk MM and AA pts.
We incorporated a series of measures to decrease GI toxicity, infections and cardiac failure to our ASCT program. All pts receive amifostine 740 mg/m2 as a bolus infusion before MEL. In addition, stem cell collection is performed inpatient if pts are on hemodialysis (HD) and/or have high-risk AA. Peripheral blood stem cell (PBSC) mobilization uses plerixafor and Neupogen. The CardioMEMS HF System is used for high-risk AA with cardiac involvement to achieve more accurate fluid balance throughout the ASCT process. This device is FDA-approved for wirelessly measuring and monitoring pulmonary artery pressure and heart rate in New York Heart Association Class III heart failure pts who have been hospitalized for heart failure in the previous year. HD was done on the day before and the day of transplant and three times weekly thereafter. DMSO used for PBSC cryopreservation is washed from the infused stem cell product prior to infusion. High-risk pts are those older than age 65 years, on HD, or with high-risk AA (Mayo stage III, Kumar et al. JCO 30.9 (2012): 989-995). All pts fulfilling these criteria treated from January 2013 to Dec 2016 were retrospectively analyzed. Survival outcomes were measured from the date of ASCT until disease relapse or progression, Survival distribution was estimated using Kaplan-Meier methods.
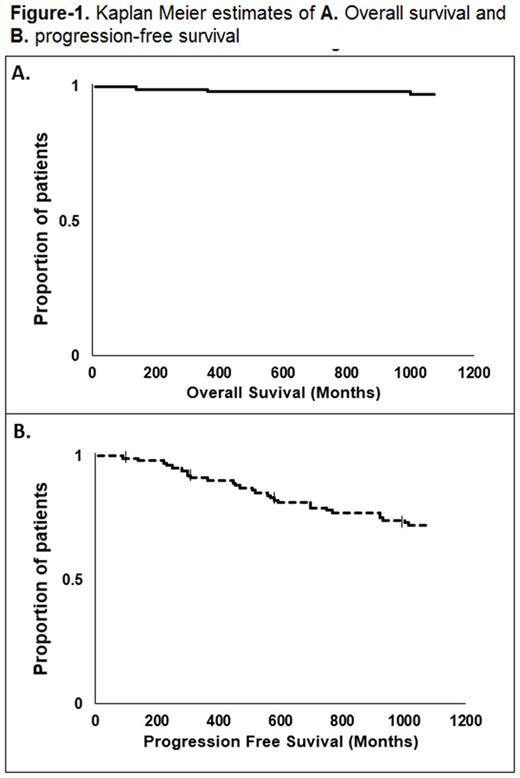
Thirty seven pts were analyzed (median age=66.6 years; range: 45-77). Thirty-five pts (95%) were older > 65. Fourteen pts were female (37%). Five pts were on HD (14%); two had AA with cardiac involvement. Six pts had light chain disease (16%), 21 IgG (56%) and eight had IgA (21%) MM, and two had AL AA. Five pts were ISS stage I (14%), 16 stage II (46%) and 14 stage III (40%). Five MM pts (14%) had extramedullary disease. Thirty pts (85%) were considered standard-risk and 5 had high-risk cytogenetics (14%). Median time from diagnosis to ASCT was 8.7 months (range: 3.2-169.4). Median number of therapy lines was 1 (range 1-7). Thirty pts (85%) had at least partial response (PR) at the time of transplant. Nine pts (24%) were collected with filgrastim only and 28 (75%) with filgrastim and Plerixafor (including all pts on HD and AA). Median number of CD34 cells collected was 6.36 x10E6/Kg (range: 2.34-23.99 x10E6/Kg). Median number of CD34 cells infused was 3.25 x10E6/Kg (range: 1.88-10.8). Fourteen pts (37%) received conditioning with MEL 140 mg/m2, and 63% received 200 mg/m2. Median length of hospital stay for ASCT was 15 days (range: 13-25). Grade II-III toxicity was seen in 13 pts (35%), with only 2 grade III and no grade IV GI toxicity. There was no difference in toxicity between MEL 140 vs. 200 (P=0.210). Median length of mucositis was 6.5 days (range: 2-18). One pt used PCA dilaudid. No pt needed total parenteral nutrition. Median time to neutrophil and platelet engraftment was 11 (range: 9-15) and 12 days (range: 8- 19), respectively. Transplant-related mortality was zero. Day 100 response rate (at least PR) occurred in 34 pts (97%), with 18 (51%) achieving near complete remission or better. Twenty eight (75%) received maintenance therapy. Median follow-up time for survivors was 38 months. At the end of follow up period, only 4 pts (10%) had died. Median PFS was not reached, 3-year PFS: 70% (Figure-1.A and B).
Taken together, our results indicate the safety of the sequential approach described here. Prospective studies to examine the use of CardioMEMs and amifostine to improve clinical outcome of high-risk autologous transplant are warranted.
Cooper: Novartis: Research Funding. Caimi: Abbvie: Equity Ownership; Incyte: Equity Ownership; Seattle Genetics: Equity Ownership; Celgene: Speakers Bureau. de Lima: Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Research Funding. Malek: Sanofi: Membership on an entity's Board of Directors or advisory committees; Celgene: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.